peteb
Elite Cafe Member
Here are some photos of a "breadboard" layout of my anodizer setup. It is useful because it pinpoints areas for coloring, reducing the need to mask everything. It
is very simple to use: place the work in the container in contact with the screen, add electrolite, depress the safety switch with one hand and guide the wand at an oblique angle over the area to be colored.
Happy to answer any questions. <br>


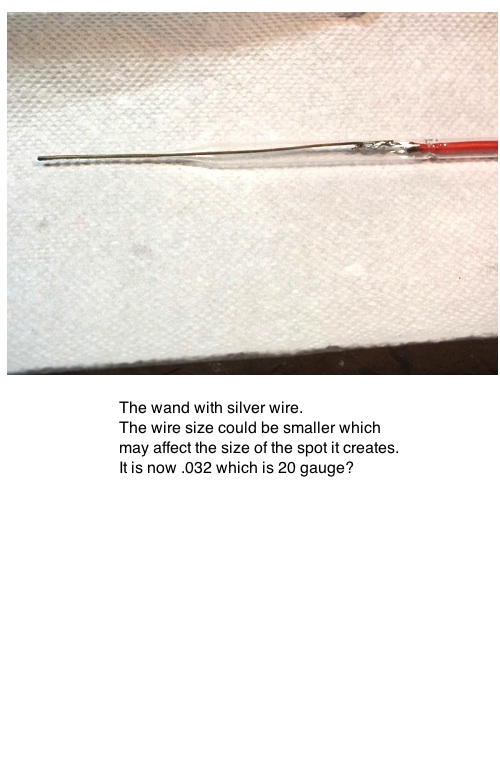

is very simple to use: place the work in the container in contact with the screen, add electrolite, depress the safety switch with one hand and guide the wand at an oblique angle over the area to be colored.
Happy to answer any questions. <br>






